
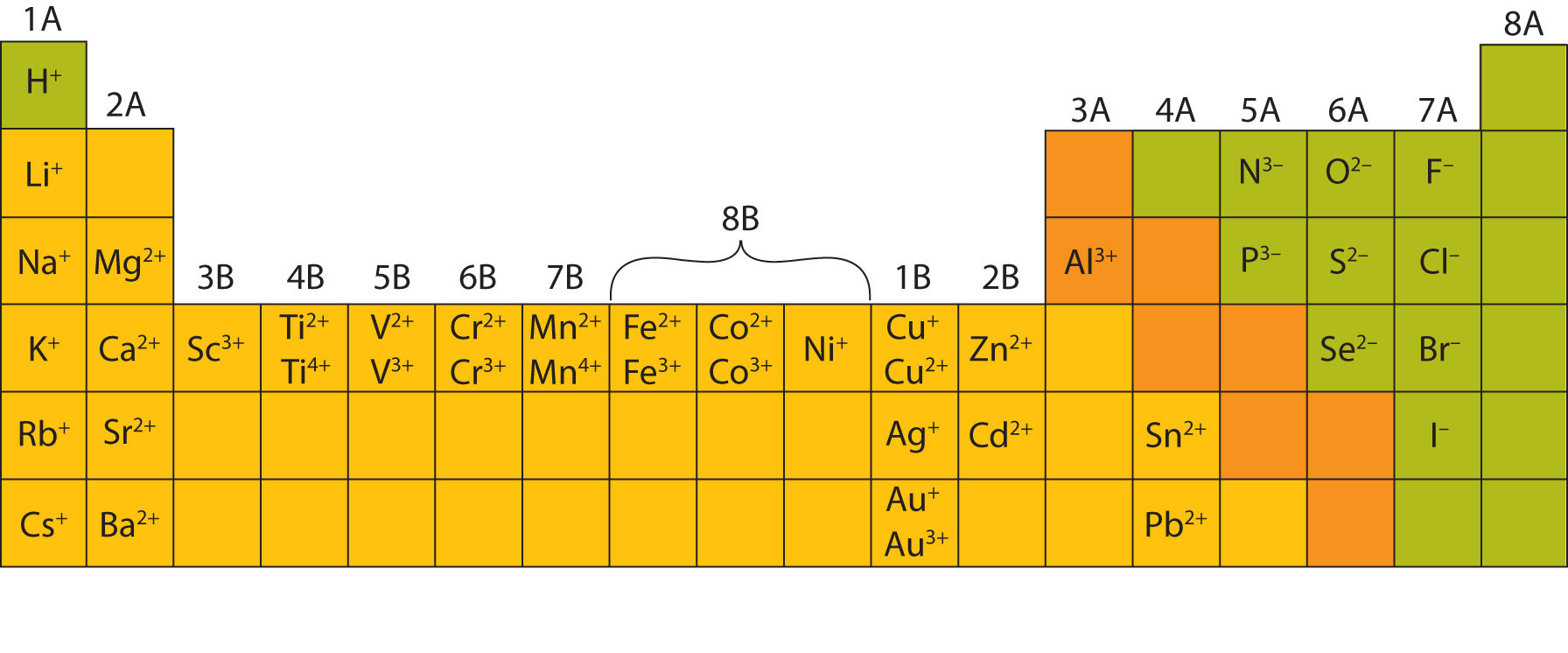
These elements are metals that form oxides that have an earthy texture and yield alkaline solutions. By contrast, consider the alkaline earths in the second column on the left side of the table. Charges are balanced in a compound with the number of alkali metal ions equal to the number of halogen ions NaCl is such a compound. The alkali metals, on the other side of the periodic table, all readily lose one electron, so their ions possess a charge of +1. The halogens all tend to gain one electron, giving their ions a characteristic charge of –1. In the periodic table, elements in a column form analogous compounds because they have the same charge on their ions. The chemical behavior of the various elements is influenced more by the charge of their ion than by any other intrinsic property. Table 1 shows three different charge states for copper: A positive ion has lost one or more electrons, whereas a negative ion has gained one or more electrons.

Atoms with a charge imbalance are called ions. A neutral atom has precisely equal numbers of protons (+) and electrons (–). Of the three major subatomic particles, the negatively charged electron was first discovered by the English physicist Joseph Thomson in 1897.Īs the structure of atoms was probed, it was realized that these low‐mass particles occurred in a large “cloud” around the tiny nucleus, which contained almost all the mass of the atom. Quiz: Introduction to Oxidation-Reduction Reactions.Introduction to Oxidation-Reduction Reactions.Quiz: Heat Capacities and Transformations.Quiz: Introduction to Organic Compounds.Quiz: Compounds with Additional Elements.


 0 kommentar(er)
0 kommentar(er)
